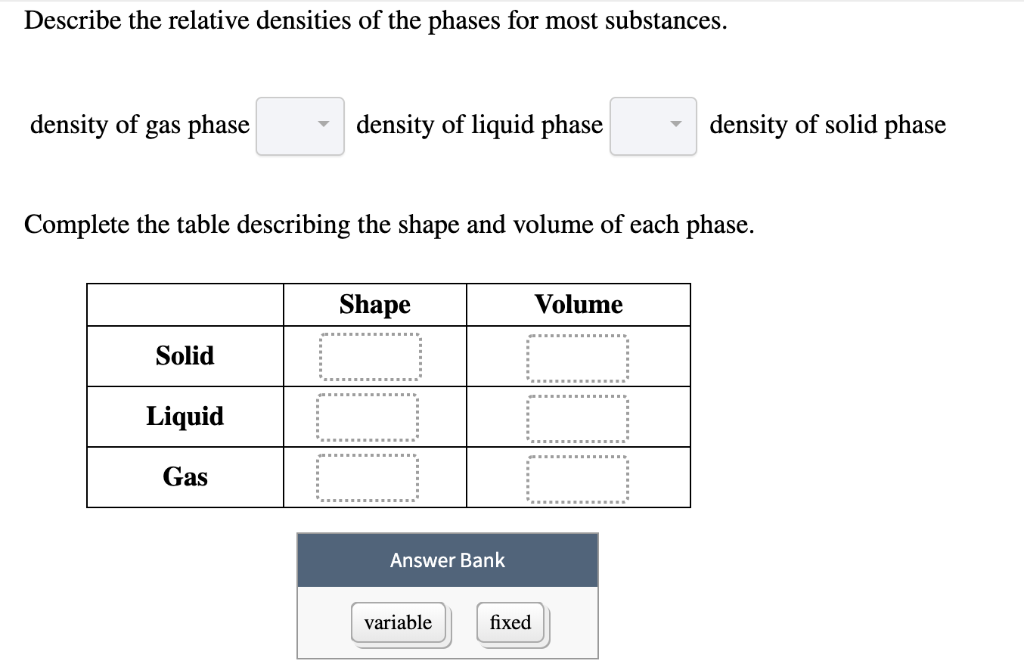
Describe the Relative Densities of the Phases for Most Substances
Introduced in 1949 the Redlich-Kwong RK equation was a substantial improvement with respect to other equation of its time. An effort has been made to include the compounds most frequently encountered in the laboratory the workplace and the environment.
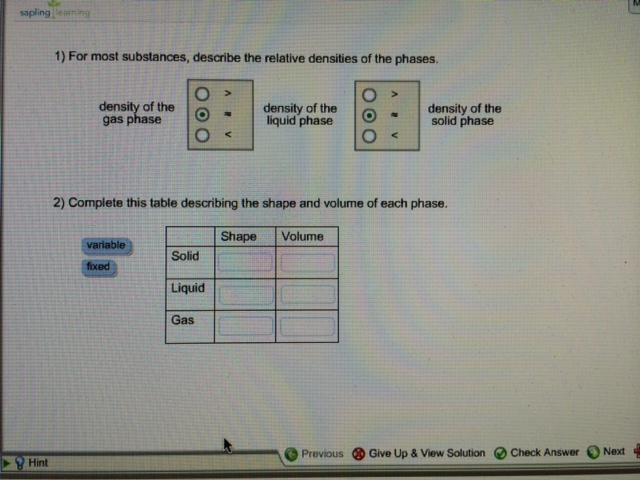
Solved For Most Substances Describe The Relative Densities Chegg Com
The sun is an average-sized yellow star about 110 times the diameter of Earth.
. When moving from the bottom of the diagram to the top the. Moving along a constant temperature line reveals relative densities of the phases. The solid-liquid curve exhibits a positive slope indicating that the melting point for CO 2 increases with pressure as it does for most substances water being a notable exception as described previously.
Elemental carbon has one gas phase one liquid phase and two different solid phases as shown in the phase diagram. B Graphite is the most stable phase of carbon at normal conditions. The density of solids and liquids normally increase with decreasing temperature.
Some of the most widely used equations of state are. Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. Its monatomic form H is the most abundant chemical substance in the Universe constituting.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. The phases of the moon include the new waxing crescent first quarter waxing gibbous full waning gibbous last third quarter and waning crescent. A On the phase diagram label the gas and liquid regions.
Notice that the triple point is well above 1 atm indicating that carbon dioxide. With most substances the temperature and pressure related to the triple point lie below standard temperature and pressure and the pressure for the critical point lies above standard pressure. It is still focus of attention due to its relative simple expression.
Particular emphasis has been given to substances that are considered environmental or human health hazards. The phases of the moon are caused by its position relative to Earth and the sun. SUN MOON EARTH -- SIZE MAKEUP.
The basic physical constants and structure diagrams for 10867 organic compounds are presented in this table. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. The densities of the solids and liquids displayed are given for the standard temperature of 00 C 00 C and the densities of solids and liquids depend on the temperature.
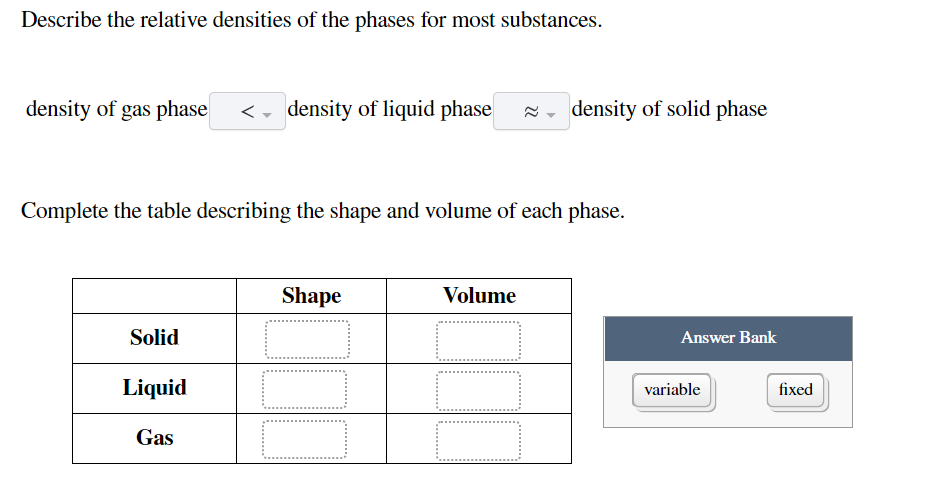
Solved Describe The Relative Densities Of The Phases For Chegg Com
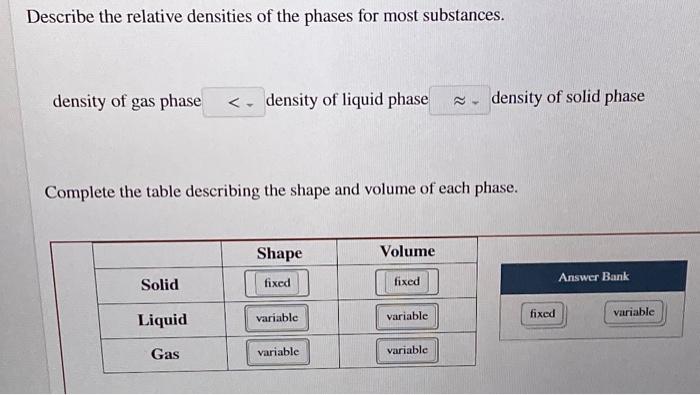
Solved Describe The Relative Densities Of The Phases For Chegg Com
0 Response to "Describe the Relative Densities of the Phases for Most Substances"
Post a Comment